Diffusion and Osmosis - Biology - Naba
Hello there fellow gobs, today I will become a teacher *😎* and teach you about diffusion and osmosis.
Firstly, what is diffusion?
Diffusion is the net movement of particles from a region of higher concentration to a lower concentration down the concentration gradient as a result of random movement.
Too confusing? Here, let's simplify it.
In image one, you'll see that particles have been concentrated on the left corner of the box and after sometime in the second box, the molecules have spread out.
What's a concentration gradient?
A concentration gradient is basically a slope from a higher concentration to a lower concentration but it's imaginary so you wouldn't really find it anywhere.
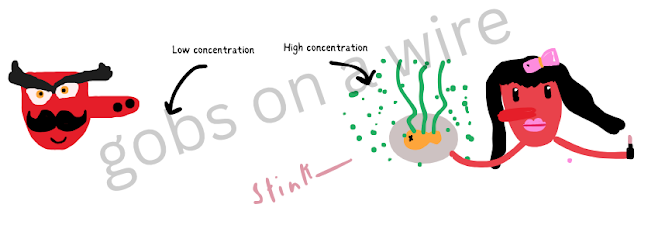
As you see, the slide down the slope to where it is less concentrated.Here's a (not) pretty example since, you know, gobs are always important -
Here, Papa Gob is happy (oblivious) though the smell of his dish is odiferous because it hasn't really reached him yet and the stink molecules are still around the fish, unknown to the chef, Mama Gob. Mama Gob is meant to smell the stink (but somehow she doesn't, not because of diffusion but perhaps she had gotten a cold.)
Now that the odour has diffused through the air, Papa Gob can smell it now because what had no concentration of yuck molecules has become evenly concentrated with them.
Soo yeah...
If diffusion is understood, then we're good to go on to osmosis and pinky-promise this isn't as hard as people make it look.
Osmosis is the diffusion of water molecules through a partially permeable membrane.
A partially permeable memberane is a memberane that allows certain molecules to pass while not allowing others to pass through.
Osmosis in terms of water potential is net movement of water molecules from a region of higher water potential to a region of lower water potential through a partialy permeable membrane.
What is water potential?Water potential is basically how dilute a solution is. If it has a high water potential, it has more water, this is a dilute solution, while a low water potential is where there is lesser water - this is a concentrated solution.
On the left side of the diagram, we see a higher water potential (dilute solution) and in the right we have a lower water potential (concentrated solution). The water molecules move from the higher water potential to the lower water potential.
Usually in diffusion, you would see a concentrated area move into a lesser concentrated area but in osmosis, it's the opposite; the dilute solution goes ahead to meet the concentrated solution. The partially permeable membrane is too small to let the sugar molecules in.
This is what it would look like on a concentration gradient.
To learn further about osmosis in plant cells, click on the link and download our "Movement in and Out of Cells" document linked below.
Until then, have a gobtastic day,
Naba.
https://drive.google.com/file/d/1RQRbrCaJJbZz81pBs5DWaxqnteVgLPHV/view?usp=sharing






this is very easy to understand, thank you fellow gobs
ReplyDelete